Istanbul Retina Institute
Dry Age-related Macular Degeneration
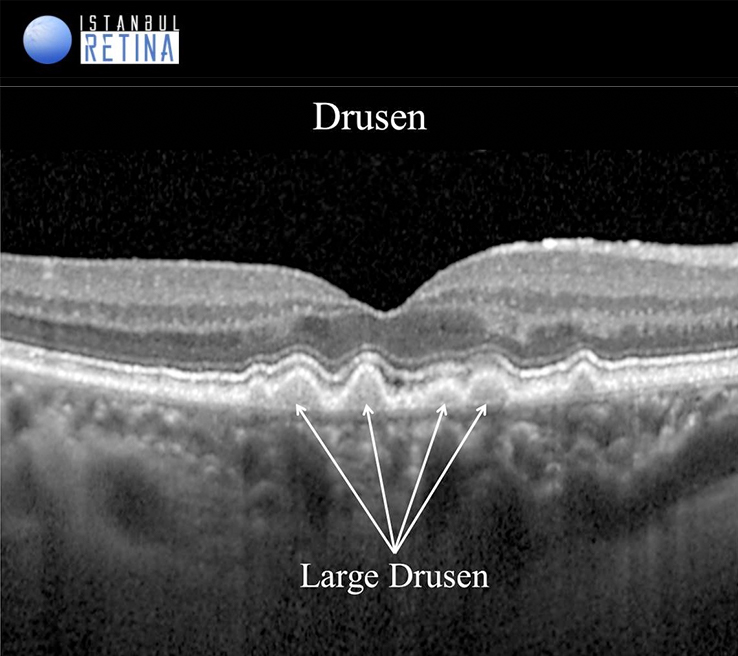
According to the latest accepted classification, observation of small (<63 mm) drusen deposits in individuals aged 55 and over is considered a normal aging process. The persons with medium-sized (63-125 µm) drusen are considered to have early age-related macular degeneration (AMD). The presence of one or more large-sized (125 µm) drusen or medium- sized (63-125 µm) drusen with pigmentary changes is interpreted as middle stage AMD. Geographic atrophy and macular neovascularization are considered advanced stage AMD.
Small, hard drusen appear on fundus photographs as well circumscribed, yellow-white deposits. On optical coherence tomography (OCT), small drusen appear as small, hyperreflective deposits localized under the retinal pigment epithelium (RPE). Soft drusen are defined as larger, poorly-defined drusen with mound-like elevations and a diameter measuring greater than 125 microns. On OCT, soft drusen appear as different-sized, dome-shaped elevations at the RPE level.
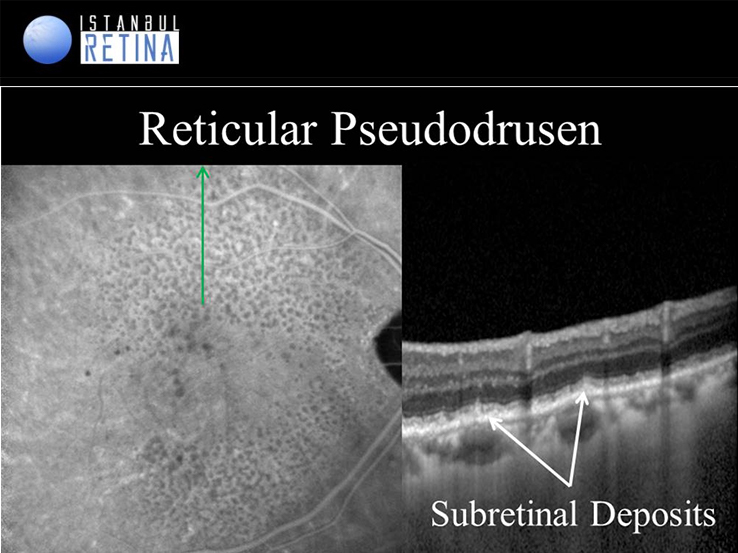
Reticular pseudodrusen or subretinal drusenoid deposits are typically localized between the sensory retina and the RPE. Reticular pseudodrusen are multiple yellowish-white lesions arranged in a reticular network pattern. On OCT scans, these lesions are shown as granular hyperreflective deposits situate between the RPE layer and the ellipsoid zone.
OCT has been used to classify reticular pseudodrusen into 3 stages. Stage 1: hyperreflective material between RPE and Inner segment-outer segment junction or ellipsoid zone (EZ). There is no elevation or breach of EZ. Stage 2: the hyperreflective material accumulates and forms a mound over the RPE with distortion of overlying EZ. Stage 3: the hyperrefelctive material has a conical configuration which punctures the EZ and reaches outer retina.
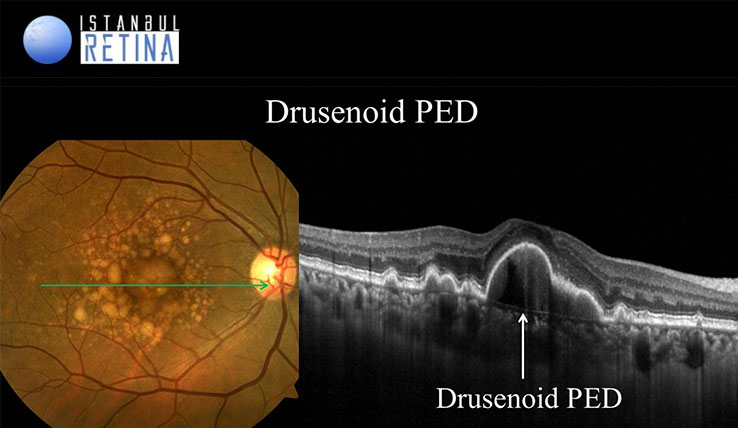
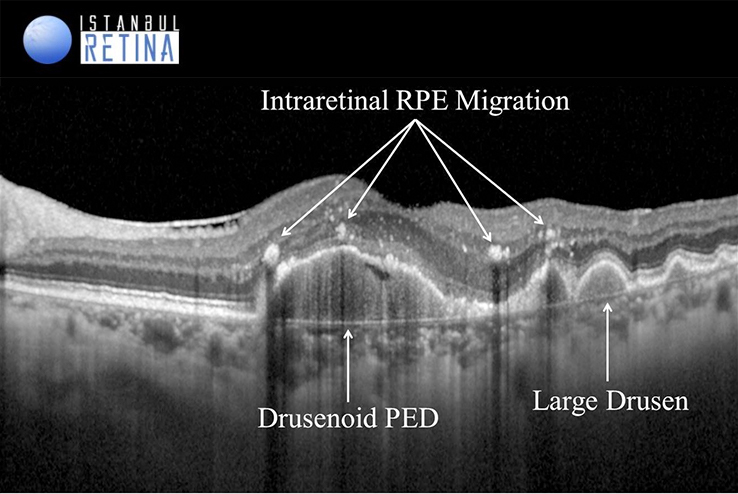
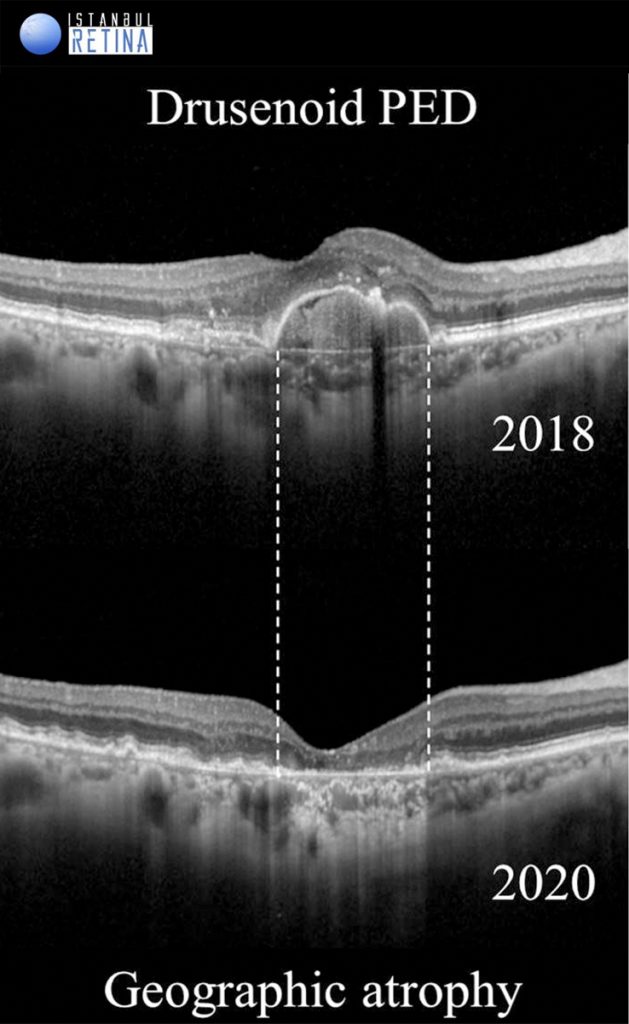
Drusenoid pigment epithelial detachments (PEDs) are yellow or yellow-white elevations of the RPE greater than 500 microns in diameter consisting of many large soft drusen or confluent drusen. On OCT, drusenoid PEDs shows dome-shaped elevation of the RPE. It may contain solid material and partially fluid, but there is no shadowing. Due to the progression of RPE loss on the drusenoid PED, it begins to regress and collapses over time, leaving an atrophic lesion.
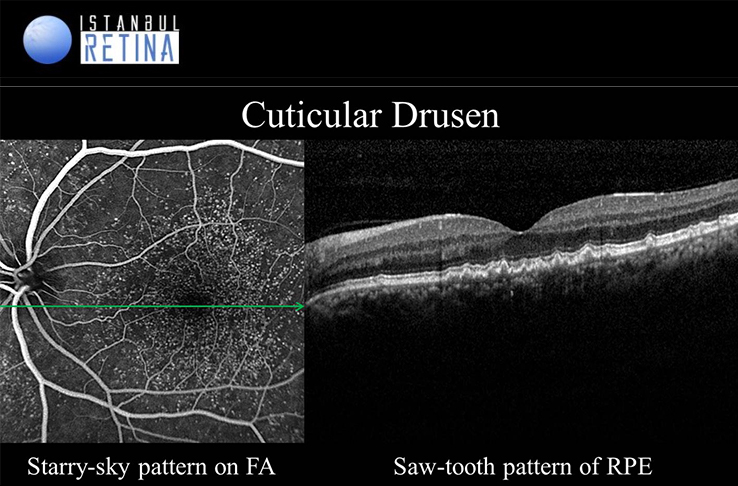
Cuticular drusen (basal laminar drusen) has recently been recognized as a specific clinical subtype of AMD. Curticular drusen, present a starry sky appearance on fluorescein angiography. SD-OCT clearly shows cuticular drusen as “saw tooth”.
Intraretinal RPE migration has been described in several retinal degenerative diseases.It is known that in response to various stimuli, RPE cells are capable of hypertrophy, proliferation, and intraretinal migration. Such changes are commonly seen in AMD and can be visualized with OCT. Intraretinal RPE migration is usually observed as intraretinal hyperreflective foci with underlying shadowing in the outer nuclear layer.
On SD-OCT, areas of geographic atrophy are characterized by loss of of RPE and increased choroidal hyperreflectivity. In addition, development of geographic atrophy is including loss of the interdigitation zone, ellipsoid zone, and external limiting membrane as well as thinning of the outer nuclear layer.
Neovascular Age-related Macular Degeneration
Neovascular AMD is classified into 3 types considering the clinical, angiographic and OCT findings:
- Type 1 Macular Neovascularization (occult)
- Type 2 Macular Neovascularization (classic)
- Type 3 Macular Neovascularization (retinal angiomatous proliferation, RAP)
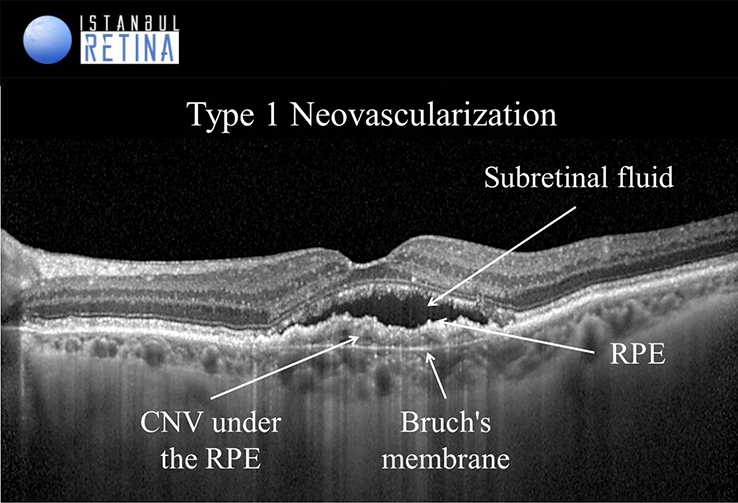
Type 1 macular neovascularization is a growth of vessels from choriocapillaris into the sub-retinal pigment epithelium space. Associated proliferation of fibrotic tissue can cause expansion of a fibrovascular PED. Fibrovascular PEDs are present in 62–80% of eyes with neovascular AMD. Fibrovascular PED in type 1 macular neovascularization appears as an irregular RPE elevation with or without serous exudate. SD-OCT may also reveal the choroidal neovascular membrane appearing as a hyperreflective material on the posterior surface of the lesion. The presence of hyporeflective subretinal or intraretinal fluid as well as hyperreflective exudate and hemorrhage is interpreted as active neovascularization.
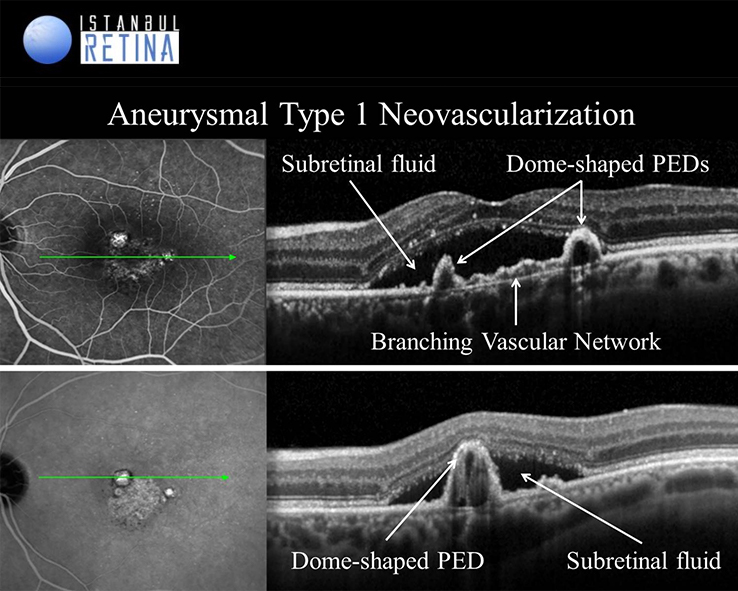
Type-1 aneurysmal neovascularization (Polypoidal Choroidal Vasculopathy) is considered a variant of neovascular AMD. Polypoidal choroidal vasculopathy PCV is a disease of the inner choroidal vasculature. In OCT images, the branching vascular network is observed as shallow irregular PED (double-layer sign), while polypoidal lesions are observed as dome-shaped or round structures. In addition, PCV may present with spontaneous massive subretinal hemorrhage, hemorrhagic and serous PED.
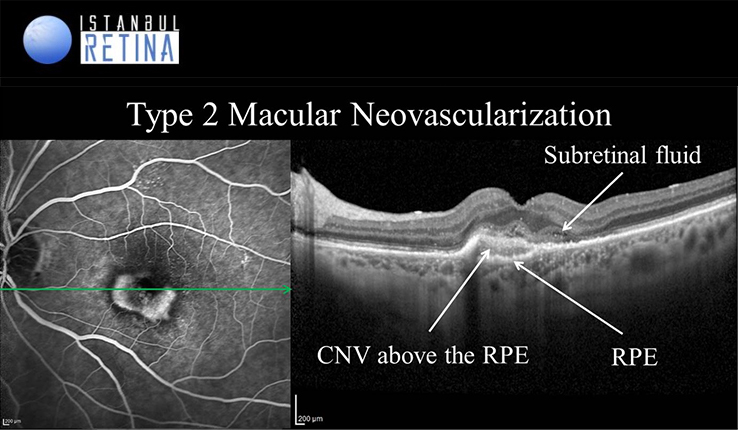
Type 2 neovascularization accounts for approximately 10% of newly diagnosed macular neovascularizations. Classic macular neovascularization appears on OCT as hyperreflective material located above the RPE. The presence of intraretinal and subretinal fluid, intraretinal cysts, ellipsoid zone edema is considered as a sign of active disease. OCT images can also show increased intraretinal or subretinal reflectivity consistent with hemorrhage and lipid accumulation.
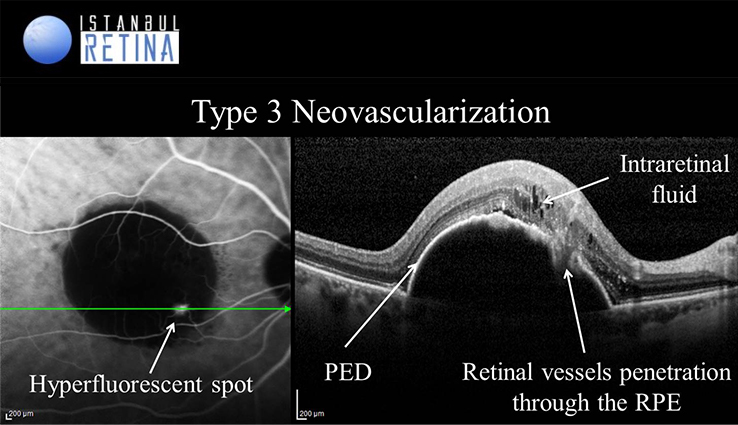
Type 3 neovascularization or RAP accounts for approximately 15% of newly diagnosed macular neovascularizations. It is a bilateral disease and involvement with up to 100% of the fellow eye within 3 years has been reported. It is more common in women. Reticular pseudodrusen is reported to be a risk factor for the development of RAP. RAP involves 3 stages.
In stage I (intraretinal neovascularization) vascular proliferation originates from the deep capillary plexus of the retina in the paramacular area and is confined within the retina, as a retinal-retinal anastomosis. Intraretinal haemorrhage and edema are common. In stage II (subretinal neovascularization) neovascular proliferation invades the subretinal space. Neurosensory and serous PED can be seen, together with macular edema and pre-retinal or intraretinal haemorrhages. In stage III choroidal neovascularization is present. It can be associated with vascularized pigment PED. A retinal-choroidal anastomosis is formed.
- Imaging features of type 3 neovascularization are variable.
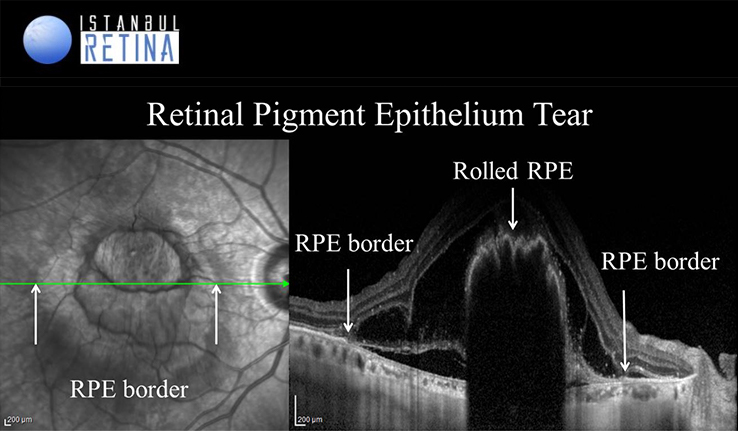
Retinal pigment epithelium tears represent a serious complication in patients with neovascular AMD and frequently results in profound loss of central vision. The most common cause of a RPE tear is vascularized PED. Spontaneous tear rate of vascular PEDs of 10-13% has been reported. Large PED basal diameter and vertical height are correlated with an increased risk of developing an RPE tear. OCT is the most valuable diagnostic tool for RPE tear. The rolled RPE has irregular contour and causes a hyperreflectivity with an intense back-shadowing masking the choroid. The area of RPE loss may demonstrate an increased depth signal due to the absent RPE monolayer. The neurosensory retina appears intact over the tear, with or without subretinal fluid.
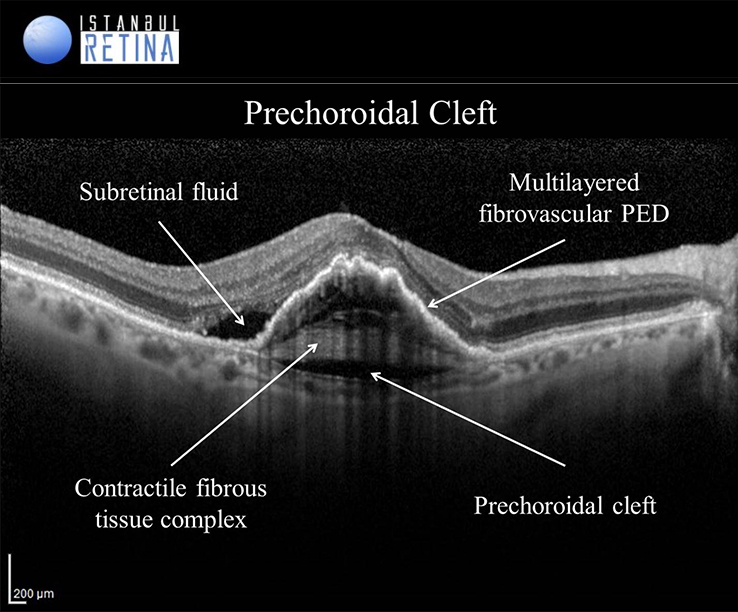
Prechoroidal cleft is an OCT finding observed in patients with neovascular AMD that is characterized by a hyporeflective space between hyperreflective materials in PED and Bruch’s membrane. This finding has been postulated to indicate residual activities and some contractile forces of the sub-RPE fibrovascular tissue.
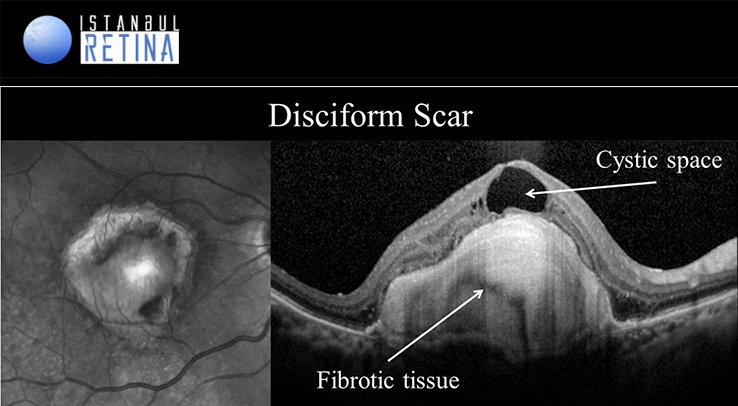
Disciform scars form the stage the end-stage of neovascular AMD. Over time in untreated neovascular AMD the vascular component typically regresses, while the fibrous component increases, resulting in disciform scar formation. Disciform scar formation appears on OCT as a well demarcated highly hyperreflective lesion. Subretinal scaring damages the RPE and photoreceptors resulting in irreversible vision loss.
References:
Spaide RF, Jaffe GJ, Sarraf D, et. Consensus Nomenclature for Reporting Neovascular Age-Related Macular Degeneration Data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group. Ophthalmology. 2020;127(5):616-636.
Keane PA, Patel PJ, Liakopoulos S, et al. Evaluation of age-related macular degeneration with optical coherence tomography. Surv Ophthalmol 2012;57:389-414.
Schmidt-Erfurth U, Klimscha S, Waldstein SM, Bogunović H. A view of the current and future role of optical coherence tomography in the management of age-related macular degeneration. Eye (Lond). 2017;31:26-44.
Ferris FL 3rd, Wilkinson CP, Bird A, Chakravarthy U, Chew E, Csaky K, Sadda SR; Beckman Initiative for Macular Research Classification Committee.Clinical classification of age-related macular degeneration. Ophthalmology 2013;120(4):844-51.
Hocaoğlu M, Arf S, Karaçorlu M. Drusen Çeşitleri ve Görüntülenme Yöntemleri. Güncel Retina 2017;1:116-123.
Sayman Muslubaş I, Arf S, Karaçorlu M. Yaş Tip (Neovasküler) Yaşa Bağlı Makula Dejenerasyonunda Sınıflandırma ve Tanı. Güncel Retina 2017;1(3):189-197.
Ersoz MG, Karacorlu M, Arf S, Sayman Muslubas I, Hocaoglu M. Retinal pigment epithelium tears: Classification, pathogenesis, predictors, and management. Surv Ophthalmol 2017;62(4):493-505.
Mukai R, Sato T, Kishi S. A hyporeflective space between hyperreflective materials in pigment epithelial detachment and Bruch’s membrane in neovascular age-related macular degeneration. BMC Ophthalmol 2014;14:159.