Istanbul Retina Institute
Enhanced-depth imaging optical coherence tomography (EDI-OCT) and swept-source OCT have enabled in vivo visualization of the outer margin of the choroid. The term pachychoroid originates from the Greek παχύ, or pachy, which means thick. It is used to describe pachychoroid spectrum diseases that exhibit some common choroidal features. The spectrum of pachychoroid disorders is characterized by increased choroidal thickness (diffuse or focal), dilation of outer choroidal vessels (pachyvessels), and attenuation and thinning of the choriocapillaris and the middle choroidal vessels overlying these pachyvessels.
Common Clinical Features of the Pachychoroid Disorders
Choroidal Thickening
The normal subfoveal choroidal thickness (SFCT) has been reported in the range of 190-350 μm. Although, there is no particular threshold value of SFCT for thick choroid. In normal eyes the thickest choroidal area is subfoveal. While choroid thickness varies in many different studies, subfoveal choroidal thickness of > 300 μm, > 350 μm and 395 μm was considered as thick choroid. Increased choroidal thickening may be focal or diffuse. Eyes with pachychoroid disease may have normal subfoveal choroidal thickness, but exhibit an extrafoveal focus of increased choroidal thickness (defined as exceeding subfoveal choroidal thickness by 50 µm). The area of maximal choroidal thickness is likely to be of significance if it correlates spatially with the distribution of pachyvessels and with the disease focus.
Pachyvessels
The dilation of choroidal vessels in the Haller’s layer appears to account for thickened choroid in pachychoroid spectrum disorders. The choroidal vessels in the Haller’s layer are pathologically dilated (pachyvessels) and are observed as hyporeflective lumen with large diameter on OCT. Additionally, there is focal or diffuse attenuation in Sattler’s layer and choriocapillaris. Due to thinning of the inner choroidal structures, the choroidal thickness can be measured as normal. Regardless of choroidal thickness, presence of morphological features of pathologic sequelae resulting from abnormally dilated choroid may be a more significant finding for diagnosing pachychoroid disease. Retinal pigment epithelium (RPE) lesions, choroidal neovascularization (CNV) and polyps observed in pachychoroid spectrum diseases typically overlie these choroidal abnormalities.
Choroidal Hyperpermeability
Hyperfluorescent patches seen in middle and late phases of indocyanine green angiography (ICGA) are defined as choroidal hyperpermeability. Choroidal hyperpermeability over 90% in eyes with pachychoroid pigment epitheliopathy (PPE) and central serous chorioretinopathy (CSCR), and %50 in eyes with uncomplicated pachychoroid has been reported. Complications associated with pachychoroid are often observed in areas with choroidal hyperpermeability. Choroidal hyperperemability on ICGA is also observed in the areas of lekage on fluorescein angiography (FA). However, areas of choroidal hyperpermeability on ICGA are usually larger than the areas of leakage on FA.
CLASSIFICATION
The following entities are included in the spectrum of pachychoroid disorders:
- Uncomplicated pachychoroid (UCP)
- Pachychoroid pigment epitheliopathy (PPE)
- Central serous chorioretinopathy (CSCR)
- Pachychoroid neovasculopathy (PNV)
- Aneurysmal type 1 neovascularization (AT1N) or polypoidal choroidal vasculopathy (PCV)
- Peripapillary pachychoroid syndrome (PPS)
- Focal choroidal excavation (FCE)
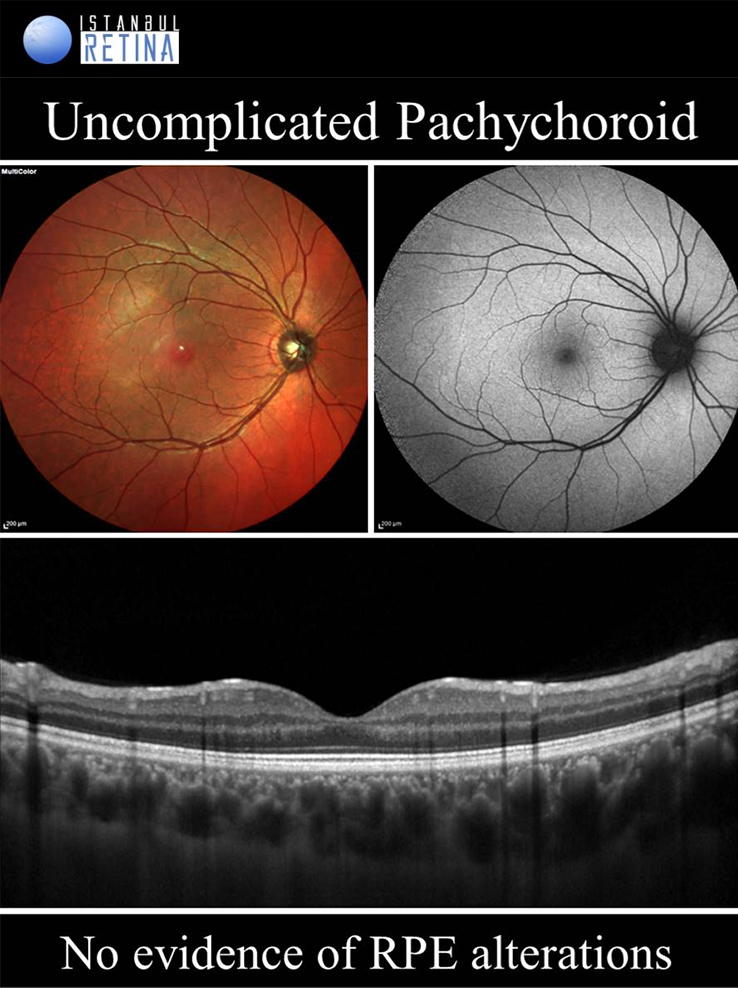
Uncomplicated Pachychoroid
If only pachychoroid characteristics are observed, but there is no RPE changes, CNV or polyps, then eyes are classified as having an uncomplicated pachychoroid. Uncomplicated pachychoroid is not a pathological condition. This term has been used in clinical studies to describe the uncomplicated fellow eyes of patients with pachycoroid spectrum diseases.
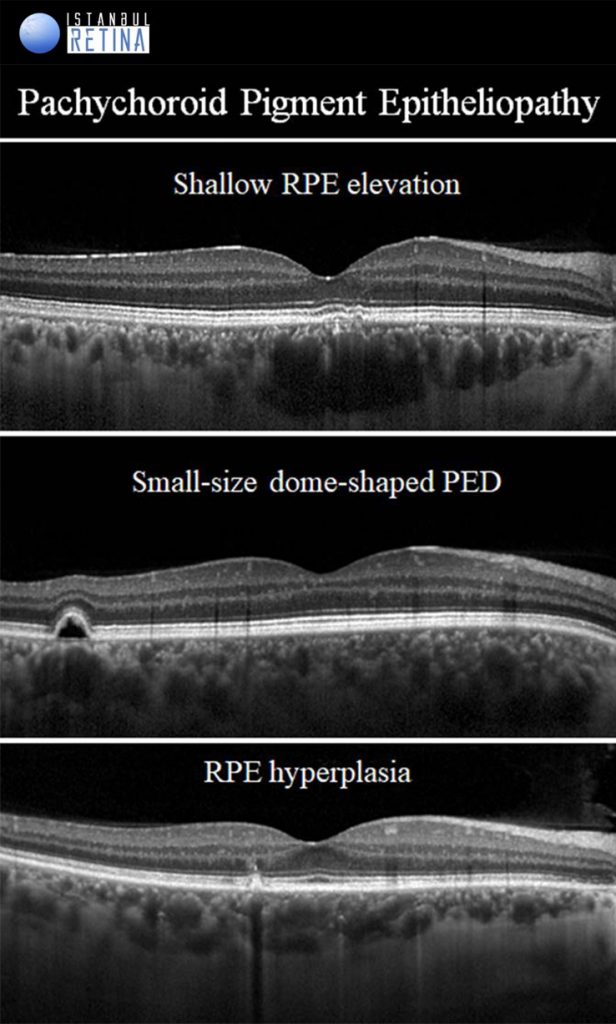
Pachychoroid Pigment Epitheliopathy
If only pachychoroid features and RPE chages are observed, but there is no subretinal fluid, CNV or polyps, then eyes are classified as having a PPE. Pachychoroid pigment epitheliopathy refers to a precursor or forme fruste of CSCR. Pachychoroid pigment epitheliopathy can ultimately progress to the development of CSCR or PNV. The RPE lesions that can be observed in eyes with PPE are: microbreak, RPE thickening, hyperreflective spike of RPE, pigment epithelial detachment (PED) and pachydrusen.
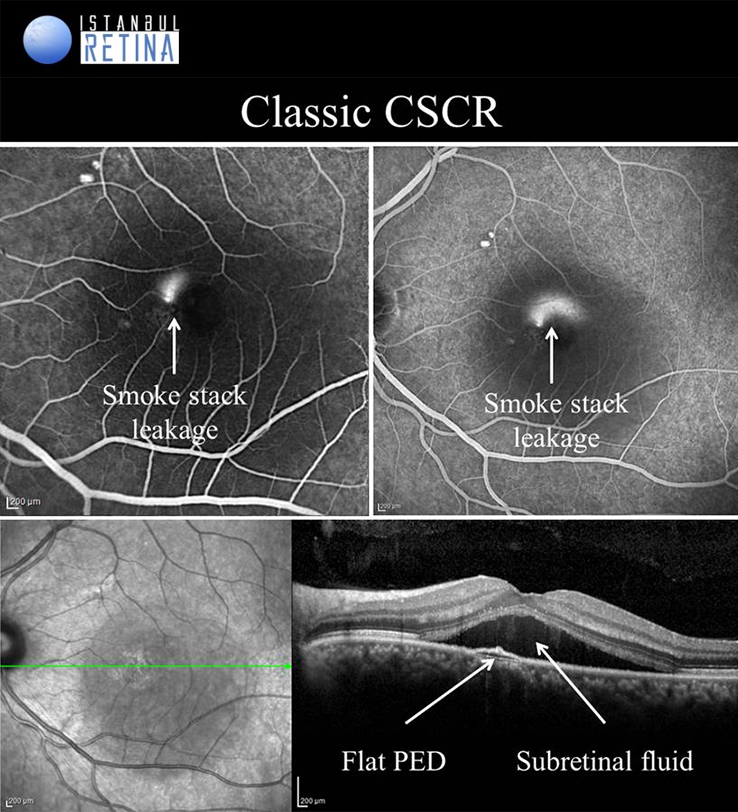
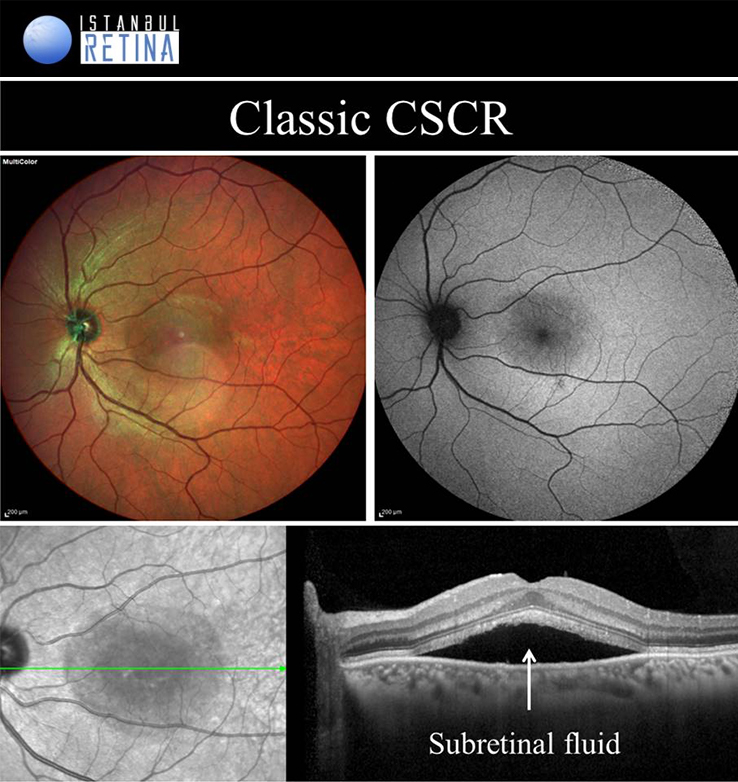
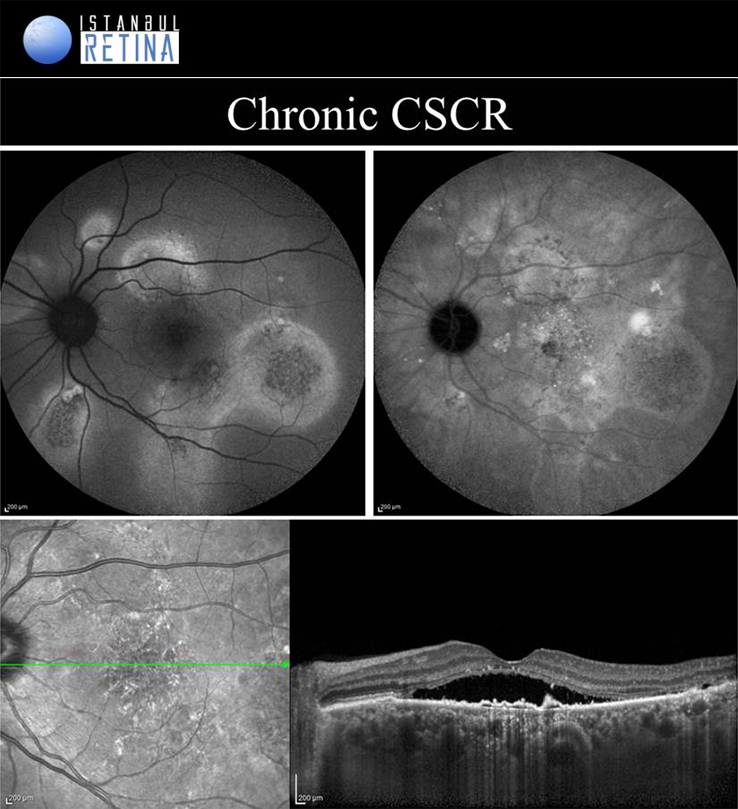
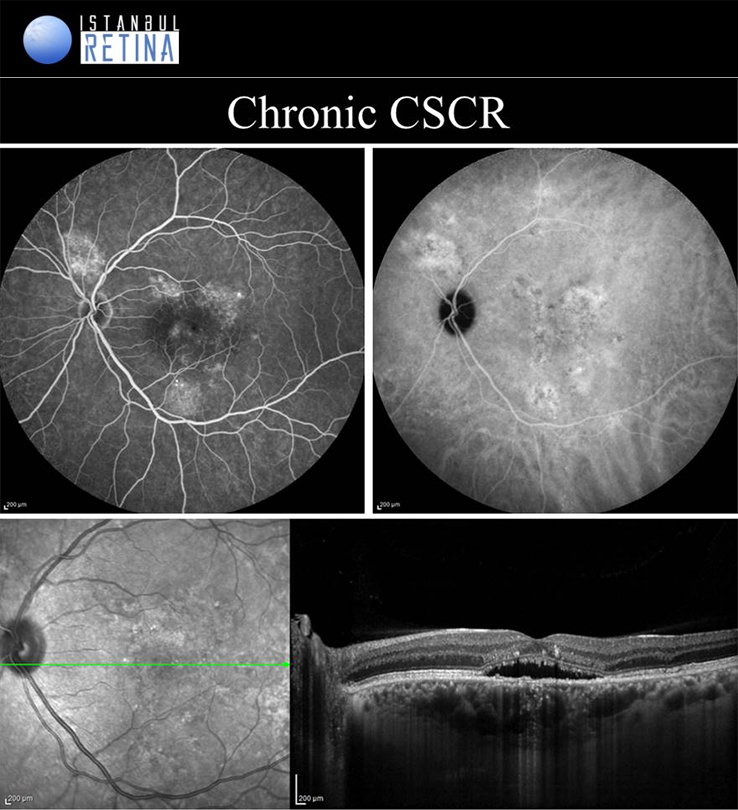
Central Serous Chorioretinopathy
If pachychoroid characteristics and subretinal fluid are seen, but there is no CNV or polyps, then eyes are classified as having a CSCR. Central serous chorioretinopathy can ultimately progress to the development of PCV or PNV. Several classifications describing the CSCR subtypes have been proposed. The “acute CSCR’’ is characterized by limited RPE changes, subretinal fluid and leakage at the level of the RPE. Most cases of acute CSCR are self-limiting and regress spontaneously within 3–4 months. In general, acute and chronic forms are distinguished based on the duration of first attack of CSCR. However, there is no consensus regarding the duration. The patients with persistent serous detachment on OCT for longer than 4–6 months are defined as having “chronic CSCR’’ or “persistent CSCR”. The term ‘’recurrent CSCR’’ is considered when subretinal fluid resolves spontaneously after more than one attack of CSCR.
In some cases, CSCR episodes are limited to the extramacular area. Because they are asymptomatic, typically go undetected. Due to the presence asymptomatic patients, it may not be correct to distinguish according to duration. Many authors use a basic distinction based on the degree of RPE alteration. Patients with limited areas of RPE changes, one or few leakage areas on FA, and small amount of subretinal fluid are diagnosed as “classical CSCR”. On the other hand, if fundus autofluorescence demonstrates widespread RPE atrophy, they are described as having “chronic CSCR”.
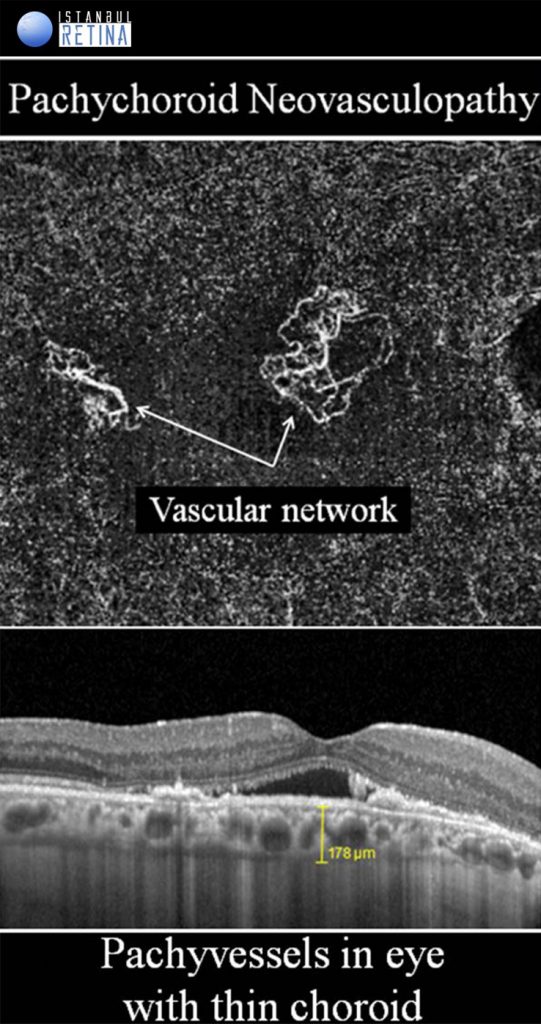
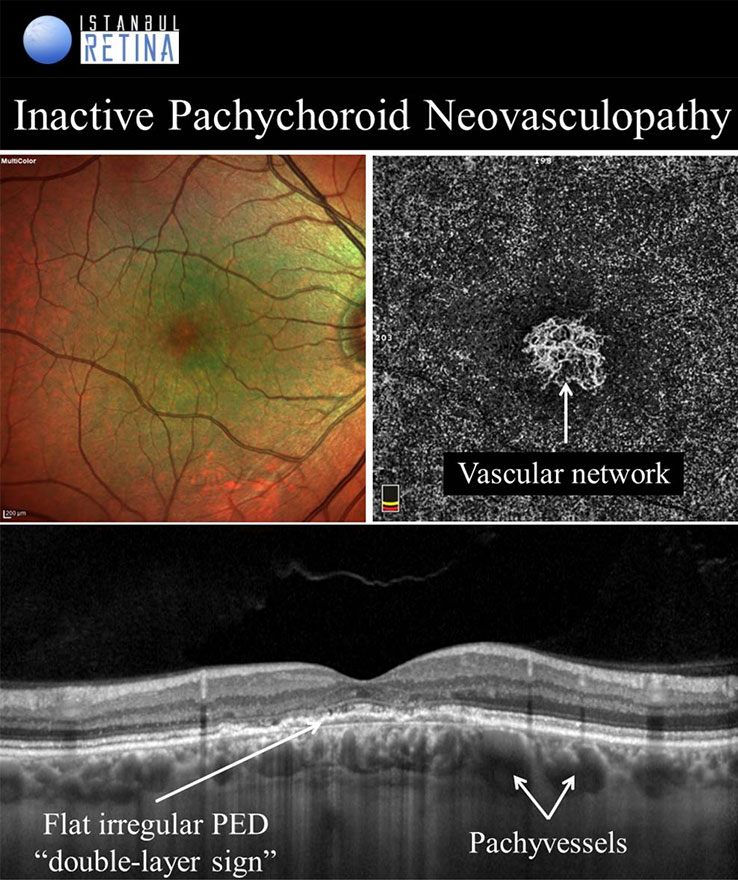
Pachychoroid Neovasculopathy
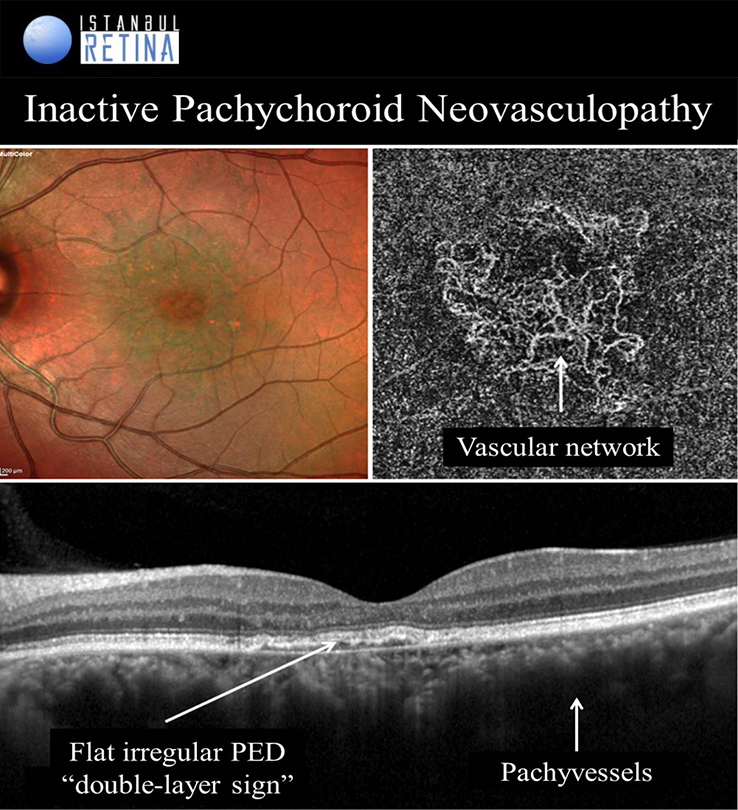
It is well known that CNV and PCV may develop in eyes with CSCR. Pachychoroid neovasculopathy is characterized by type-1 choroidal neovascularization without polyps, overlying focal areas of choroidal thickening and dilated choroidal vessels. The PPE and CSCR spectrum of pachychoroid diseases may progress to PNV. Progression of PNV to aneurysmal pachychoroid neovasculopathy (PCV) was also demonstrated. Type-1 CNV was detected in %74-95 of pachychoroid eyes with shallow irregular PEDs using OCTA. Pachychoroid neovasculopathy may appear as active or inactive macular neovascularization. It is thought that 11% of PNVs are inactive lesions. CNV associated with chronic CSCR may be type 1 or type 2. Type 2 CNV in pachychoroid eyes is a distinct condition and a standardized definition has not yet been established.
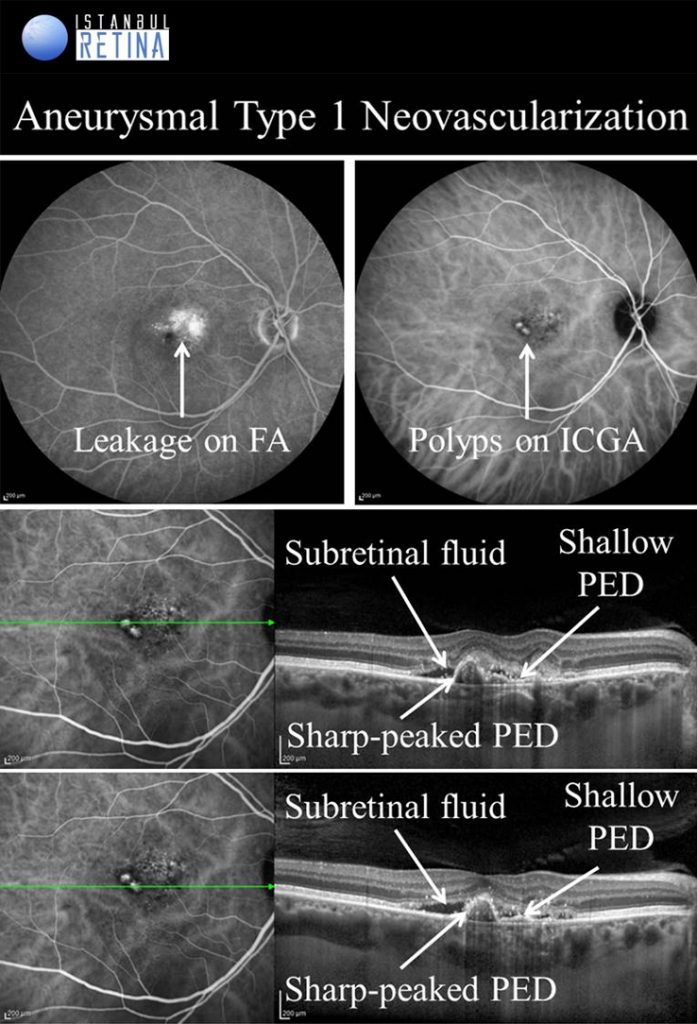
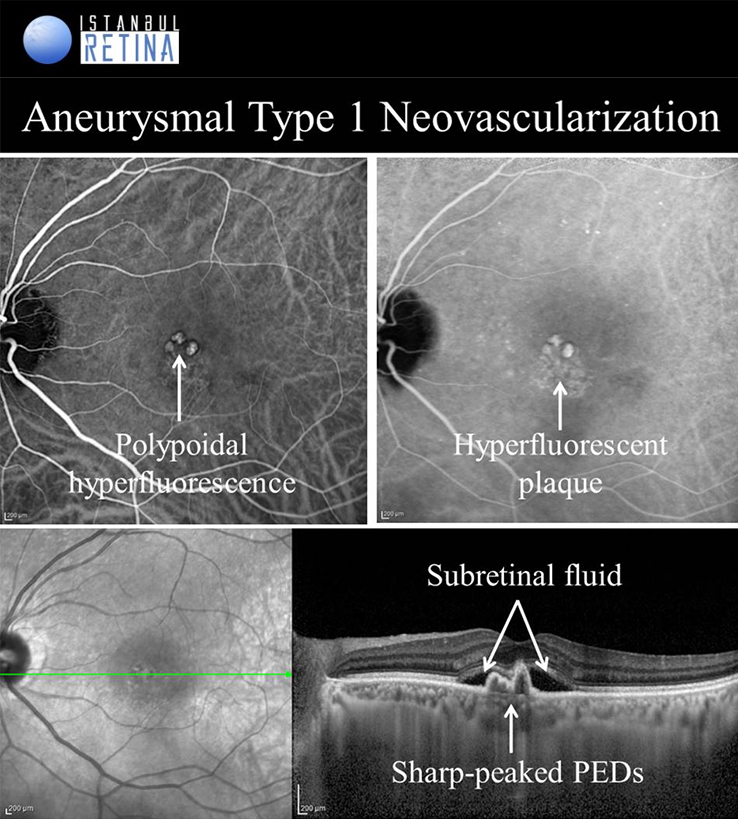
Aneurysmal Type 1 Novascularization
Aneurysmal type 1 neovascularization (polypoidal choroidal vasculopathy PCV) was defined as a peaked PED (corresponding to the aneurysmal lesion) adjacent to a shallow irregular PED (corresponding to type 1 neovascularization). Aneurysmal type 1 novascularization has also been described in various pathological conditions including CSCR, circumscribed choroidal hemangioma, melanocytoma of the optic nerve, pathological myopia and staphyloma, choroidal osteoma, peripheral exudative hemorrhagic chorioretinopathy and choroidal nevi. The term polypoidal choroidal vasculopathy was introduced by Yannuzzi et al. in 1990. This entity was described as a degenerative disease featuring exudative and haemorrhagic pigment epithelial and neurosensory detachments. In 1995, Spaide et al. published an analysis of ICGA performed on patients with PCV and defined PCV by the presence of branching vascular network and polypoidal structures. Yuzawa et al. classified PCV into two types. PCV type 1 was characterized by the presence of feeder vessels and draining venules. PCV type 2 was defined as vascular lesions caused by inner choroidal vessel abnormalities, not by CNV. Most recently, PCV was described as a part of the pachychoroid spectrum, characterised by thick choroid, dilated choroidal vessels in Haller layer, attenuation of the choriocapillaris and Sattler’s layer and loss of fundus tessellation.
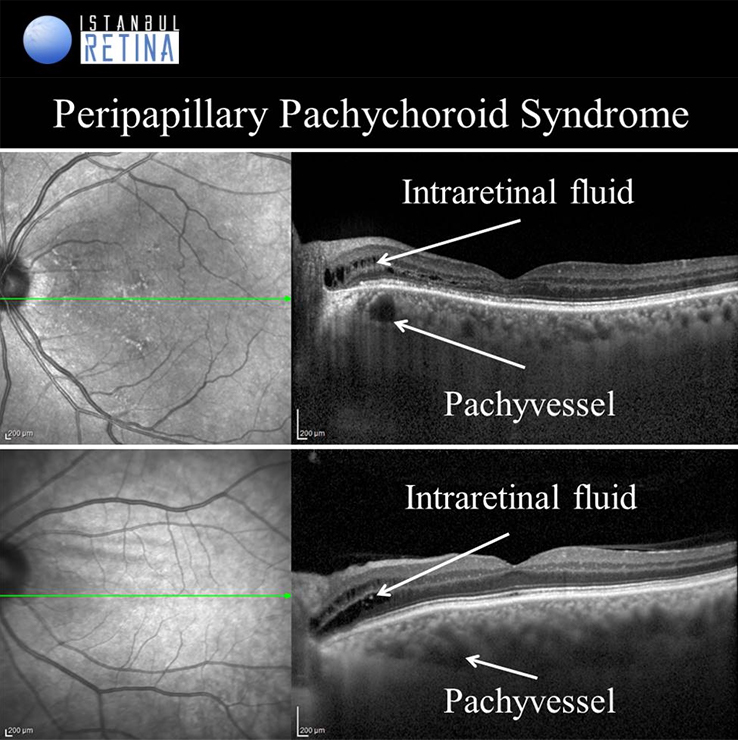
Peripapillary Pachychoroid Syndrome
Peripapillary pachychoroid syndrome is a novel pachychoroid disease spectrum variant, in which peripapillary choroidal thickening is associated with intraretinal and/or subretinal fluid.
Peripapillary atrophy and intraretinal fluid around the optic disc is observed in all patients. Subretinal fluid may develop in some patients. Choroidal folds, short axial length, hyperopia, crowded disc and leakage of the optic nerve head are among the typical findings for FCE.
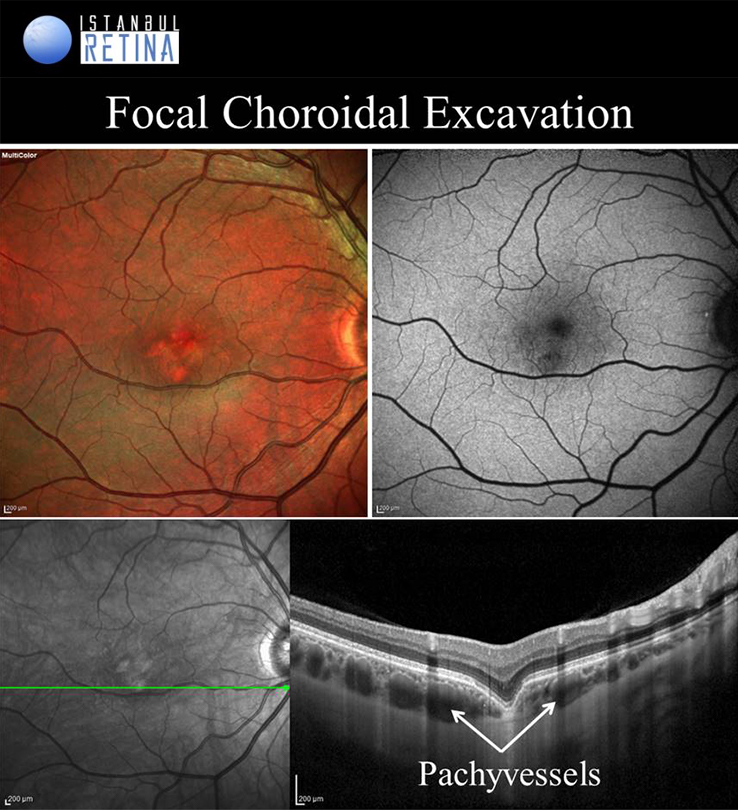
Focal Choroidal Excavation
Focal choroidal excavation is described as a localised area of choroidal excavation without any evidence of posterior staphyloma or scleral ectasia. Choroidal excavations have been divided into either the conforming type in which there is no separation between the photoreceptor layer and the RPE, or the non-conforming type in which the photoreceptor layer appear to be detached from the underlying RPE. Based on another classification,
FCE might occur as cone-shaped, bowl-shaped type the mixed type in OCT. Focal choroidal excavation is believed to be part of the pachychoroid spectrum. While the exact pathogenesis of FCE remains unclear, it has been proposed that in some cases there is an association with the inflammation in the outer retina.
References:
Ersoz MG, Hocaoğlu M. Pakikoroid Spektrum Hastalıklarına Genel Bakış ve Sınıflama. Güncel Retina 2020; 4(2): 67-74.
Karacorlu M. Pakikoroid Pigment Epitelyopati; Bulgular, Tanı ve Yaklaşım. Güncel Retina 2020; 4(2): 93-97.
Arf S, Hocaoğlu M, Ersöz MG. Santral Seröz Koryoretinopati, Pakikoroid Spektrumu Hastalıklar ve Koroid Neovaskülarizasyonları. Güncel Retina 2017;1(4):289-295.
Ersoz MG, Arf S, Hocaoglu M, Sayman Muslubas I, Karacorlu M. Patient characteristics and risk factors for central serous chorioretinopathy: an analysis of 811 patients. Br J Ophthalmol. 2019;103(6):725-729.
Ersoz MG, Arf S, Hocaoglu M, Sayman Muslubas I, Karacorlu M. Indocyanine Green Angıography Of Pachychoroid Pigment Epıtheliopathy. Retina. 2018;38(9):1668-1674.
Ersoz MG, Karacorlu M, Arf S, Hocaoglu M, Sayman Muslubas I. Outer Nuclear Layer Thinning In Pachychoroid Pigment Epitheliopathy. Retina. 2018 May;38(5):957-961.
Ersoz MG, Karacorlu M, Arf S, Hocaoglu M, Sayman Muslubas I. Pachychoroid pigment epitheliopathy in fellow eyes of patients with unilateral central serous chorioretinopathy. Br J Ophthalmol. 2018;102(4):473-478.
Arf S, Hocaoglu M, Sayman Muslubas I, Karacorlu M. Efficacy of reduced-fluence photodynamic therapy for central serous chorioretinopathy associated with combined serous retinal detachment and fovea-involving pigment epithelial detachment. Int Ophthalmol. 2017;37(3):483-489.
Muslubas IS, Ersoz MG, Hocaoglu M, Arf S, Karacorlu M. Morphological and Functional Changes Immediately After Half-Time Photodynamic Therapy in Patients With Central Serous Chorioretinopathy. Ophthalmic Surg Lasers Imaging Retina. 2018 Dec 1;49(12):932-940.
Sayman Muslubaş I, Hocaoğlu M, Arf S, Özdemir H, Karaçorlu M. Treatment Outcomes in Patients with Polypoidal Choroidal Vasculopathy. Turk J Ophthalmol. 2016;46(1):16-20.
Cheung CMG, Lee WK, Koizumi H, et al. Pachychoroid disease. Eye (Lond). 2019;33(1):14-33.